Sodium borohydride
 | |||||||||||||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||||||||||||
| Names | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Preferred IUPAC name Sodium tetrahydridoborate (1–) | |||||||||||||||||||||||||||||||||||||||||||
Systematic IUPAC name Sodium boranuide | |||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||
CAS Number |
| ||||||||||||||||||||||||||||||||||||||||||
3D model (JSmol) |
| ||||||||||||||||||||||||||||||||||||||||||
ChEBI |
| ||||||||||||||||||||||||||||||||||||||||||
ChemSpider |
| ||||||||||||||||||||||||||||||||||||||||||
ECHA InfoCard | 100.037.262 | ||||||||||||||||||||||||||||||||||||||||||
EC Number | 241-004-4 | ||||||||||||||||||||||||||||||||||||||||||
Gmelin Reference | 23167 | ||||||||||||||||||||||||||||||||||||||||||
MeSH | Sodium+borohydride | ||||||||||||||||||||||||||||||||||||||||||
PubChem CID |
| ||||||||||||||||||||||||||||||||||||||||||
RTECS number | ED3325000 | ||||||||||||||||||||||||||||||||||||||||||
UN number | 1426 | ||||||||||||||||||||||||||||||||||||||||||
InChI
| |||||||||||||||||||||||||||||||||||||||||||
SMILES
| |||||||||||||||||||||||||||||||||||||||||||
| Properties | |||||||||||||||||||||||||||||||||||||||||||
Chemical formula | NaBH4 | ||||||||||||||||||||||||||||||||||||||||||
Molar mass | 37.83 g/mol | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | white crystals hygroscopic | ||||||||||||||||||||||||||||||||||||||||||
Density | 1.0740 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||
Melting point | 400 °C (752 °F; 673 K)[1] | ||||||||||||||||||||||||||||||||||||||||||
Boiling point | 500 °C (932 °F; 773 K) (decomposes)[1] | ||||||||||||||||||||||||||||||||||||||||||
Solubility in water | soluble, reacts with water | ||||||||||||||||||||||||||||||||||||||||||
Solubility | soluble in liquid ammonia, amines, pyridine | ||||||||||||||||||||||||||||||||||||||||||
| Hazards | |||||||||||||||||||||||||||||||||||||||||||
GHS hazard statements | H260, H301, H311, H314 | ||||||||||||||||||||||||||||||||||||||||||
GHS precautionary statements | P223, P231, P232, P280, P301+310, P370+378, P422 | ||||||||||||||||||||||||||||||||||||||||||
NFPA 704 | Flash point 70 °C (158 °F; 343 K) | Autoignition temperature ca. 220 °C (428 °F; 493 K) | Explosive limits 3% | Lethal dose or concentration (LD, LC): | LD50 (median dose) 160 mg/kg (Oral - Rat) | 230 mg/kg (Dermal - Rabbit) Related compounds | Other anions Sodium cyanoborohydride Sodium hydride Sodium borate Borax Other cations Lithium borohydride Related compounds Lithium aluminium hydride Sodium triacetoxyborohydride Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate,[2] is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a reducing agent that finds application in chemistry, both in the laboratory and on a technical scale. It has been tested as pretreatment for pulping of wood, but is too costly to be commercialized.[3][4] The compound is soluble in alcohols, certain ethers, and even water, although it slowly hydrolyzes[5] The compound was discovered in the 1940s by H. I. Schlesinger, who led a team seeking volatile uranium compounds.[6] Results of this wartime research were declassified and published in 1953. Contents
| ||||||||||||||||||||||||||||||||||||
| Solvent | Solubility (g/100 mL)[5] |
|---|---|
| MeOH | 13 |
| EtOH | 3.16 |
| Diglyme | 5.15 |
| Et2O | insoluble |
Sodium borohydride is an odorless white to gray-white microcrystalline powder that often forms lumps. It can be purified by recrystallization from warm (50 °C) diglyme.[7] Sodium borohydride is soluble in protic solvents such as water and lower alcohols. It also reacts with these protic solvents to produce H2, however, these reactions are fairly slow. Complete decomposition of a methanol solution requires nearly 90 min at 20 °C.[8] It will decompose in neutral or acidic aqueous solutions, but is stable at pH 14.[5]
Structure
NaBH4 is a salt, consisting of the tetrahedral BH4− anion. The solid is known to exist as three polymorphs: α, β and γ. The stable phase at room temperature and pressure is α-NaBH4, which is cubic and adopts an NaCl-type structure, in the Fm3m space group. At a pressure of 6.3 GPa, the structure changes to the tetragonal β-NaBH4 (space group P421c) and at 8.9 GPa, the orthorhombic γ-NaBH4 (space group Pnma) becomes the most stable.[9][10][11]
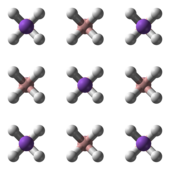 | 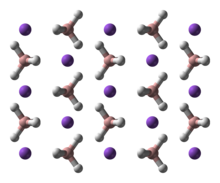 | 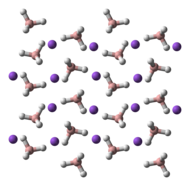 |
α-NaBH4 | β-NaBH4 | γ-NaBH4 |
Synthesis and handling
For commercial NaBH4 production, the Brown-Schlesinger process and the Bayer process are the most popular methods. In the Brown-Schlesinger process Sodium borohydride is industrially prepared following the original method of Schlesinger: sodium hydride (produced by reacting Na and H2) is treated with trimethyl borate at 250–270 °C:
- B(OCH3)3 + 4 NaH → NaBH4 + 3 NaOCH3
Millions of kilograms are produced annually, far exceeding the production levels of any other hydride reducing agent.[3] Sodium borohydride can also be produced by the action of NaH on powdered borosilicate glass.[12]
Different from it, the Bayer process is based on the reaction among borax (Na2B4O7), Na, H2, and silicon oxide (SiO2) at 700 °C to synthesize NaBH4:
- Na2B4O7 + 16 Na + 8 H2 + 7 SiO2 → 4 NaBH4 + 7 Na2SiO3
There is currently an effort to modify the Bayer Process by employing the less expensive reducing metal magnesium (Mg) in place of sodium. Reactions such as:
- 8 MgH2 + Na2B4O7 + Na2CO3 → 4 NaBH4 + 8 MgO + CO2
and
- 2 MgH2 + NaBO2 → NaBH4 + 2 MgO
are promising modifications to the Bayer Process, but have not been developed far enough to exhibit both high yield and fast reaction rates.[13][14]
Reactivity
NaBH4 will reduce many organic carbonyls, depending on the precise conditions. Most typically, it is used in the laboratory for converting ketones and aldehydes to alcohols. It will efficiently reduce acyl chlorides, anhydrides, α-hydroxylactones, thioesters, and imines at room temperature or below. It will reduce esters slowly and inefficiently with excess reagent and/or elevated temperatures, while carboxylic acids and amides are not reduced at all.[15] NaBH4 reacts with water and alcohols, with evolution of hydrogen gas and formation of the borate salt, the reaction being especially fast at low pH.
Nevertheless, an alcohol, often methanol or ethanol, is generally the solvent of choice for sodium borohydride reductions of ketones and aldehydes. The mechanism of ketone and aldehyde reduction has been scrutinized by kinetic studies, and contrary to popular depictions in textbooks, the mechanism does not involve a 4-membered transition state like alkene hydroboration,[16] or a six-membered transition state involving a molecule of the alcohol solvent.[17] Hydrogen-bonding activation is required, as no reduction occurs in an aprotic solvent like diglyme. However, the rate order in alcohol is 1.5, while carbonyl compound and borohydride are both first order, suggesting a mechanism more complex than one involving a six-membered transition state that includes only a single alcohol molecule. It was suggested that the simultaneous activation of the carbonyl compound and borohydride occurs, via interaction with the alcohol and alkoxide ion, respectively, and that the reaction proceeds through an open transition state.[18][19]
α,β-Unsaturated ketones tend to be reduced by NaBH4 in a 1,4-sense, although mixtures are often formed. Addition of cerium chloride as an additive greatly improves the selectivity for 1,2-reduction of unsaturated ketones (Luche reduction). α,β-Unsaturated esters also undergo 1,4-reduction in the presence of NaBH4.[5]
Many other hydride reagents are more strongly reducing. These usually involve replacing hydride with alkyl groups, such as lithium triethylborohydride and L-Selectride (lithium tri-sec-butylborohydride), or replacing B with Al. Variations in the counterion also affect the reactivity of the borohydride.[20]
The reactivity of NaBH4 can be enhanced or augmented by a variety of compounds.[21][22] Oxidation with iodine in tetrahydrofuran gives the borane–tetrahydrofuran complex, which can reduce carboxylic acids.[23] Likewise, the NaBH4-MeOH system, formed by the addition of methanol to sodium borohydride in refluxing THF, reduces esters to the corresponding alcohols.[24] Mixing water or an alcohol with the borohydride converts some of it into unstable hydride ester, which is more efficient at reduction, but the reductant will eventually decompose spontaneously to give hydrogen gas and borates. The same reaction can run also intramolecularly: an α-ketoester converts into a diol, since the alcohol produced will attack the borohydride to produce an ester of the borohydride, which then reduces the neighboring ester.[25] The combination of NaBH4 with carboxylic acids results in the formation of acyloxyborohydride species, such as STAB. These can perform a variety of reductions not normally associated with borohydride chemistry, such as alcohols to hydrocarbons and nitriles to primary amines.[26]
Coordination chemistry
BH4− is a ligand for metal ions. Such borohydride complexes are often prepared by the action of NaBH4 (or the LiBH4) on the corresponding metal halide. One example is the titanocene derivative:[27]
- 2 (C5H5)2TiCl2 + 4 NaBH4 → 2 (C5H5)2TiBH4 + 4 NaCl + B2H6 + H2
Hydrogen source
In the presence of metal catalysts, sodium borohydride releases hydrogen. Exploiting this reactivity, sodium borohydride is used in prototypes of the direct borohydride fuel cell. The hydrogen is generated for a fuel cell by catalytic decomposition of the aqueous borohydride solution:
- NaBH4 + 2 H2O → NaBO2 + 4 H2 (ΔH < 0)
Applications
The principal application of sodium borohydride is the production of sodium dithionite from sulfur dioxide: Sodium dithionite is used as a bleaching agent for wood pulp and in the dyeing industry.
Sodium borohydride reduces aldehydes and ketones to give the related alcohols. This reaction is used in the production of various antibiotics including chloramphenicol, dihydrostreptomycin, and thiophenicol. Various steroids and vitamin A are prepared using sodium borohydride in at least one step.
Sodium borohydride is used as reducing agent in the synthesis of gold nanoparticles.[28]
Sodium borohydride has been considered as a solid state hydrogen storage candidate. Although practical temperatures and pressures for hydrogen storage have not been achieved, in 2012 a core–shell nanostructure of sodium borohydride was used successfully to store, release and reabsorb hydrogen under moderate conditions.[29]
Sodium borohydride can be used to reduce foxing in old books and documents.[30]
Safety
Sodium borohydride is a source of basic borate salts which can be corrosive, and hydrogen or diborane, which are both flammable. Spontaneous ignition can result from solution of sodium borohydride in dimethylformamide.
See also
- Lithium borohydride
- Lithium aluminium hydride
- Diisobutylaluminium hydride
References
^ ab MSDS data (carl roth)
^ Busch, D.H. (2009). Inorganic Syntheses. 20. Wiley. p. 137. ISBN 9780470132869. Retrieved 20 May 2015..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ ab Peter Rittmeyer, Ulrich Wietelmann "Hydrides" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_199
^ Istek, A. and Gonteki, E. "Utilization of sodium borohydride (NaBH4) in kraft pulping process." Retrieved online on 25 September 2014 at http://www.jeb.co.in/journal_issues/200911_nov09/paper_05.pdf.
^ abcd Banfi, L.; Narisano, E.; Riva, R.; Stiasni, N.; Hiersemann, M. (2004). "Sodium Borohydride". Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons,. doi:10.1002/047084289X.rs052.CS1 maint: Uses authors parameter (link)
^ Schlesinger, H. I.; Brown, H. C.; Abraham, B.; Bond, A. C.; Davidson, N.; Finholt, A. E.; Gilbreath, J. R.; Hoekstra, H.; Horvitz, L.; Hyde, E. K.; Katz, J. J.; Knight, J.; Lad, R. A.; Mayfield, D. L.; Rapp, L.; Ritter, D. M.; Schwartz, A. M.; Sheft, I.; Tuck, L. D.; Walker, A. O. (1953). "New developments in the chemistry of diborane and the borohydrides. General summary". J. Am. Chem. Soc. 75: 186–90. doi:10.1021/ja01097a049.CS1 maint: Multiple names: authors list (link)
^ Brown, H. C. “Organic Syntheses via Boranes” John Wiley & Sons, Inc. New York: 1975.
ISBN 0-471-11280-1. page 260-1.
^ Lo, Chih-ting F.; Karan, Kunal; Davis, Boyd R. "Kinetic Studies of Reaction between Sodium Borohydride and Methanol, Water, and Their Mixtures". Industrial & Engineering Chemistry Research. 46 (17): 5478–5484. doi:10.1021/ie0608861.
^ R. S. Kumar; A. L. Cornelius (2005). "Structural transitions in NaBH[sub 4] under pressure". Appl. Phys. Lett. 87 (26): 261916. doi:10.1063/1.2158505.
^ Y. Filinchuk; A. V. Talyzin; D. Chernyshov; V. Dmitriev (2007). "High-pressure phase of NaBH4: Crystal structure from synchrotron powder diffraction data". Phys. Rev. B. 76 (9): 092104. doi:10.1103/PhysRevB.76.092104.
^ E. Kim; R. Kumar; P. F. Weck; A. L. Cornelius; M. Nicol; S. C. Vogel; J. Zhang; M. Hartl; A. C. Stowe; L. Daemen; Y. Zhao (2007). "Pressure-driven phase transitions in NaBH4: theory and experiments". J. Phys. Chem. B. 111 (50): 13873–13876. doi:10.1021/jp709840w. PMID 18031032.
^ Schubert, F.; Lang, K.; Burger, A. "Alkali metal borohydrides" (Bayer), 1960. German patent DE 1088930 19600915 (ChemAbs: 55:120851). Supplement to. to Ger. 1,067,005 (CA 55, 11778i). From the abstract: "Alkali metal borosilicates are treated with alkali metal hydrides in approx. 1:1 ratio at >100 °C with or without H pressure".
^ Review of Chemical Processes for the Synthesis of Sodium Borohydride. Millennium Cell Inc. 2004, https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/review_chemical_processes.pdf
^ Liuzhang Ouyang et al. A Recycling Hydrogen Supply System of NaBH4 Based on a Facile Regeneration Process: A Review. Inorganics 2018, 6(1), 10; https://doi.org/10.3390/inorganics6010010 , http://www.mdpi.com/2304-6740/6/1/10/pdf (free access).
^ Banfi, Luca; Narisano, Enrica; Riva, Renata; Stiasni, Nikola; Hiersemann, Martin; Yamada, Tohru; Tsubo, Tatsuyuki (2014-10-20), "Sodium Borohydride", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, pp. 1–13, doi:10.1002/047084289x.rs052.pub3, ISBN 9780470842898, retrieved 2018-07-11
^ 1937-, Carey, Francis A.,. Organic chemistry. Giuliano, Robert M., 1954- (Tenth ed.). New York, NY. ISBN 9780073511214. OCLC 915135847.
^ Marc., Loudon, G. (2009). Organic chemistry (5th ed.). Greenwood Village, Colo.: Roberts and Co. ISBN 9780981519432. OCLC 263409353.
^ Wigfield, Donald C.; Gowland, Frederick W. (March 1977). "The kinetic role of hydroxylic solvent in the reduction of ketones by sodium borohydride. New proposals for mechanism, transition state geometry, and a comment on the origin of stereoselectivity". The Journal of Organic Chemistry. 42 (6): 1108–1109. doi:10.1021/jo00426a048. ISSN 0022-3263.
^ Wigfield, Donald C. (January 1979). "Stereochemistry and mechanism of ketone reductions by hydride reagents". Tetrahedron. 35 (4): 449–462. doi:10.1016/0040-4020(79)80140-4. ISSN 0040-4020.
^ Seyden-Penne, J. "Reductions by the Alumino- and Borohydrides in Organic Synthesis"; VCH–Lavoisier: Paris, 1991.
^ Periasamy, Mariappan; Thirumalaikumar, Muniappan (2000). "Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic synthesis". Journal of Organometallic Chemistry. 609 (1–2): 137–151. doi:10.1016/S0022-328X(00)00210-2.
^ Nora de Souza, Marcus Vinícius; Alves Vasconcelos; Thatyana Rocha (1 November 2006). "Recent methodologies mediated by sodium borohydride in the reduction of different classes of compounds". Applied Organometallic Chemistry. 20 (11): 798–810. doi:10.1002/aoc.1137.
^ Kanth, J. V. Bhaskar; Periasamy, Mariappan (1 September 1991). "Selective reduction of carboxylic acids into alcohols using sodium borohydride and iodine". The Journal of Organic Chemistry. 56 (20): 5964–5965. doi:10.1021/jo00020a052.
^ da Costa, Jorge C.S.; Pais, Karla C.; Fernandes, Elisa L.; de Oliveira, Pedro S. M.; Mendonça, Jorge S.; de Souza, Marcus V. N.; Peralta, Mônica A.; Vasconcelos, Thatyana R.A. (2006). "Simple reduction of ethyl, isopropyl and benzyl aromatic esters to alcohols using sodium borohydride-methanol system" (PDF). Arkivoc: 128–133. Retrieved 29 August 2006.
^ V. Dalla, J. P. Catteau and P. Pale. Mechanistic rationale for the NaBH4 reduction of α-keto esters. Tetrahedron Letters, Volume 40, Issue 28, 9 July 1999, Pages 5193–5196.
^ W. Gribble, Gordon (1 January 1998). "Sodium borohydride in carboxylic acid media: a phenomenal reduction system". Chemical Society Reviews. 27 (6): 395. doi:10.1039/A827395Z.
^ Lucas, C. R. (1977). "Bis(5-Cyclopentadienyl) [Tetrahydroborato(1-)]Titanium". Inorganic Syntheses. 17: 93. doi:10.1002/9780470132487.ch27.
^ Low and Bansal, 2009
^ Stuart Gary, "Hydrogen storage no longer up in the air" in ABC Science 16 August 2012, citing Christian, Meganne; Aguey-Zinsou, Kondo François (2012). "Core–Shell Strategy Leading to High Reversible Hydrogen Storage Capacity for NaBH4". ACS Nano. American Chemical Society. doi:10.1021/nn3030018. Retrieved 20 August 2012.
^ Masters, Kristin. "How to Prevent and Reverse Foxing in Rare Books". bookstellyouwhy.com. Retrieved 3 April 2018.
External links
- National Pollutant Inventory – Boron and compounds
- MSDS for Sodium Borohydride
- Materials & Energy Research Institute Tokyo, Ltd.
- Chemo- and stereoselectivity using Borohydride reagents
- Material Safety Data Sheet