Electric charge
| Electric charge | |
|---|---|
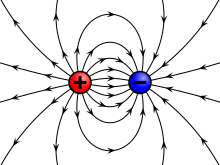 Electric field of a positive and a negative point charge | |
Common symbols | Q |
| SI unit | coulomb |
Other units |
|
| In SI base units | C = A s |
Extensive? | yes |
Conserved? | yes |
| Dimension | T I |
| Part of a series of articles about |
| Electromagnetism |
|---|
 |
|
Electrostatics
|
Magnetostatics
|
Electrodynamics
|
Electrical network
|
Covariant formulation
|
Scientists
|
Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field. There are two types of electric charges; positive and negative (commonly carried by protons and electrons respectively). Like charges repel and unlike attract. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects.
Electric charge is a conserved property; the net charge of an isolated system, the amount of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have a negative charge, if there are fewer it will have a positive charge, and if there are equal numbers it will be neutral. Charge is quantized; it comes in integer multiples of individual small units called the elementary charge, e, about 6981160200000000000♠1.602×10−19 coulombs,[1] which is the smallest charge which can exist free (particles called quarks have smaller charges, multiples of 1/3e, but they are only found in combination, and always combine to form particles with integer charge). The proton has a charge of +e, and the electron has a charge of −e.
Electric charges create an electric field, if they are moving they also generate a magnetic field. The combination of the electric and magnetic field is called the electromagnetic field, and its interaction with charges is the source of the electromagnetic force, which is one of the four fundamental forces in physics. The study of charged particles, and how their interactions are mediated by photons, is called quantum electrodynamics.
The SI derived unit of electric charge is the coulomb (C) named after French physicist Charles-Augustin de Coulomb. In electrical engineering, it is also common to use the ampere-hour (Ah); in physics and chemistry, it is common to use the elementary charge (e as a unit). Chemistry also uses the Faraday constant as the charge on a mole of electrons. The symbol Q often denotes charge.
Contents
1 Overview
2 Units
3 History
4 The role of charge in electrostatics
5 The role of charge in static electricity
5.1 Electrification by friction
6 The role of charge in electric current
7 Conservation of electric charge
8 Relativistic invariance
9 See also
10 References
11 External links
Overview
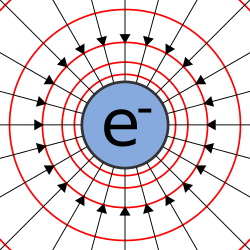
Diagram showing field lines and equipotentials around an electron, a negatively charged particle. In an electrically neutral atom, the number of electrons is equal to the number of protons (which are positively charged), resulting in a net zero overall charge
Charge is the fundamental property of forms of matter that exhibit electrostatic attraction or repulsion in the presence of other matter. Electric charge is a characteristic property of many subatomic particles. The charges of free-standing particles are integer multiples of the elementary charge e; we say that electric charge is quantized. Michael Faraday, in his electrolysis experiments, was the first to note the discrete nature of electric charge. Robert Millikan's oil drop experiment demonstrated this fact directly, and measured the elementary charge. It has been discovered that one type of particle, quarks, have fractional charges of either −1/3 or +2/3, but it is believed they always occur in multiples of integral charge; free-standing quarks have never been observed.
By convention, the charge of an electron is negative, −e, while that of a proton is positive, +e. Charged particles whose charges have the same sign repel one another, and particles whose charges have different signs attract. Coulomb's law quantifies the electrostatic force between two particles by asserting that the force is proportional to the product of their charges, and inversely proportional to the square of the distance between them. The charge of an antiparticle equals that of the corresponding particle, but with opposite sign.
The electric charge of a macroscopic object is the sum of the electric charges of the particles that make it up. This charge is often small, because matter is made of atoms, and atoms typically have equal numbers of protons and electrons, in which case their charges cancel out, yielding a net charge of zero, thus making the atom neutral.
An ion is an atom (or group of atoms) that has lost one or more electrons, giving it a net positive charge (cation), or that has gained one or more electrons, giving it a net negative charge (anion). Monatomic ions are formed from single atoms, while polyatomic ions are formed from two or more atoms that have been bonded together, in each case yielding an ion with a positive or negative net charge.
@media all and (max-width:720px).mw-parser-output .tmulti>.thumbinnerwidth:100%!important;max-width:none!important.mw-parser-output .tmulti .tsinglefloat:none!important;max-width:none!important;width:100%!important;text-align:center

During formation of macroscopic objects, constituent atoms and ions usually combine to form structures composed of neutral ionic compounds electrically bound to neutral atoms. Thus macroscopic objects tend toward being neutral overall, but macroscopic objects are rarely perfectly net neutral.
Sometimes macroscopic objects contain ions distributed throughout the material, rigidly bound in place, giving an overall net positive or negative charge to the object. Also, macroscopic objects made of conductive elements, can more or less easily (depending on the element) take on or give off electrons, and then maintain a net negative or positive charge indefinitely. When the net electric charge of an object is non-zero and motionless, the phenomenon is known as static electricity. This can easily be produced by rubbing two dissimilar materials together, such as rubbing amber with fur or glass with silk. In this way non-conductive materials can be charged to a significant degree, either positively or negatively. Charge taken from one material is moved to the other material, leaving an opposite charge of the same magnitude behind. The law of conservation of charge always applies, giving the object from which a negative charge is taken a positive charge of the same magnitude, and vice versa.
Even when an object's net charge is zero, charge can be distributed non-uniformly in the object (e.g., due to an external electromagnetic field, or bound polar molecules). In such cases the object is said to be polarized. The charge due to polarization is known as bound charge, while charge on an object produced by electrons gained or lost from outside the object is called free charge. The motion of electrons in conductive metals in a specific direction is known as electric current.
Units
The SI derived unit of quantity of electric charge is the coulomb (symbol: C). The coulomb is defined as the quantity of charge that passes through the cross section of an electrical conductor carrying one ampere for one second.[2] This unit was proposed in 1946 and ratified in 1948.[2] In modern practice, the phrase "amount of charge" is used instead of "quantity of charge".[3] The amount of charge in 1 electron (elementary charge) is approximately 6981160000000000000♠1.6×10−19 C, and 1 coulomb corresponds to the amount of charge for about 7018624000000000000♠6.24×1018 electrons. The symbol Q is often used to denote a quantity of electricity or charge. The quantity of electric charge can be directly measured with an electrometer, or indirectly measured with a ballistic galvanometer.
After finding the quantized character of charge, in 1891 George Stoney proposed the unit 'electron' for this fundamental unit of electrical charge. This was before the discovery of the particle by J. J. Thomson in 1897. The unit is today treated as nameless, referred to as elementary charge, fundamental unit of charge, or simply as e. A measure of charge should be a multiple of the elementary charge e, even if at large scales charge seems to behave as a real quantity. In some contexts it is meaningful to speak of fractions of a charge; for example in the charging of a capacitor, or in the fractional quantum Hall effect.
The unit faraday is sometimes used in electrochemistry. One faraday of charge is the magnitude of the charge of one mole of electrons,[4] i.e. 96485.33289(59) C.
In systems of units other than SI such as cgs, electric charge is expressed as combination of only three fundamental quantities (length, mass, and time), and not four, as in SI, where electric charge is a combination of length, mass, time, and electric current.[5][6]
History

Coulomb's torsion balance
From ancient times, persons were familiar with four types of phenomena that today would all be explained using the concept of electric charge: (a) lightning, (b) the torpedo fish (or electric ray), (c) St Elmo's Fire, and (d) that amber rubbed with fur would attract small, light objects.[7] The first account of the amber effect is often attributed to the ancient Greek mathematician Thales of Miletus, who lived from c. 624 – c. 546 BC, but there are doubts about whether Thales left any writings;[8] his account about amber is known from an account from early 200s.[9] This account can be taken as evidence that the phenomenon was known since at least c. 600 BC, but Thales explained this phenomenon as evidence for inanimate objects having a soul.[9] In other words, there was no indication of any conception of electric charge. More generally, the ancient Greeks did not understand the connections among these four kinds of phenomena. The Greeks observed that the charged amber buttons could attract light objects such as hair. They also found that if they rubbed the amber for long enough, they could even get an electric spark to jump,[citation needed] but there is also a claim that no mention of electric sparks appeared until late 17th century.[10] This property derives from the triboelectric effect.
In late 1100s, the substance jet, a compacted form of coal, was noted to have an amber effect,[11] and in the middle of the 1500s, Girolamo Fracastoro, discovered that diamond also showed this effect.[12] Some efforts were made by Fracastoro and others, especially Gerolamo Cardano to develop explanations for this phenomenon.[13]
In contrast to astronomy, mechanics, and optics, which had been studied quantitatively since antiquity, the start of ongoing qualitative and quantitative research into electrical phenomena can be marked with the publication of De Magnete by the English scientist William Gilbert in 1600.[14] In this book, there was a small section where Gilbert returned to the amber effect (as he called it) in addressing many of the earlier theories,[13] and coined the New Latin word electrica (from ἤλεκτρον (ēlektron), the Greek word for amber). The Latin word was translated into English as electrics.[15] Gilbert is also credited with the term electrical, while the term electricity came later, first attributed to Sir Thomas Browne in his Pseudodoxia Epidemica from 1646.[16] (For more linguistic details see Etymology of electricity.) Gilbert was followed in 1660 by Otto von Guericke, who invented what was probably the first electrostatic generator. Other European pioneers were Robert Boyle, who in 1675 stated that electric attraction and repulsion can act across a vacuum; Stephen Gray, who in 1729 classified materials as conductors and insulators. In 1733 Charles François de Cisternay du Fay, inspired by Gray's work, made a series of experiments (reported in Mémoires de l'Académie Royale des Sciences), showing that more or less all substances could be 'electrified' by rubbing, except for metals and fluids[17] and proposed that electricity comes in two varieties that cancel each other, which he expressed in terms of a two-fluid theory.[18] When glass was rubbed with silk, du Fay said that the glass was charged with vitreous electricity, and, when amber was rubbed with fur, the amber was charged with resinous electricity. Another important two-fluid theory from this time was proposed by Jean-Antoine Nollet (1745).[19] In 1839, Michael Faraday showed that the apparent division between static electricity, current electricity, and bioelectricity was incorrect, and all were a consequence of the behavior of a single kind of electricity appearing in opposite polarities. It is arbitrary which polarity is called positive and which is called negative. Positive charge can be defined as the charge left on a glass rod after being rubbed with silk.[20]
One of the foremost experts on electricity in the 18th century was Benjamin Franklin, who argued in favour of a one-fluid theory of electricity. Franklin imagined electricity as being a type of invisible fluid present in all matter; for example, he believed that it was the glass in a Leyden jar that held the accumulated charge. He posited that rubbing insulating surfaces together caused this fluid to change location, and that a flow of this fluid constitutes an electric current. He also posited that when matter contained too little of the fluid it was negatively charged, and when it had an excess it was positively charged. For a reason that was not recorded, he identified the term positive with vitreous electricity and negative with resinous electricity. William Watson independently arrived at the same explanation at about the same time (1746).
It is now known that the Franklin–Watson model was fundamentally correct. There is only one kind of electrical charge, and only one variable is required to keep track of the amount of charge.[21] On the other hand, just knowing the charge is not a complete description of the situation. Matter is composed of several kinds of electrically charged particles, and these particles have many properties, not just charge.
The role of charge in electrostatics
This section may be confusing or unclear to readers. In particular, section title was created in a guess about the intention of this paragraph, maybe this material needs two or more sections. (April 2018) (Learn how and when to remove this template message) |
All bodies are electrified, but may appear not electrified because of the relatively similar charge of neighboring objects in the environment. An object further electrified + or – creates an equivalent or opposite charge by default in neighboring objects, until those charges can equalize. The effects of attraction can be observed in high-voltage experiments, while lower voltage effects are merely weaker and therefore less obvious. Coulomb's law has a corollary for acceleration in a gravitational field. See also Casimir effect.
The role of charge in static electricity
Static electricity refers to the electric charge of an object and the related electrostatic discharge when two objects are brought together that are not at equilibrium. An electrostatic discharge creates a change in the charge of each of the two objects.
Electrification by friction
When a piece of glass and a piece of resin—neither of which exhibit any electrical properties—are rubbed together and left with the rubbed surfaces in contact, they still exhibit no electrical properties. When separated, they attract each other.
A second piece of glass rubbed with a second piece of resin, then separated and suspended near the former pieces of glass and resin causes these phenomena:
- The two pieces of glass repel each other.
- Each piece of glass attracts each piece of resin.
- The two pieces of resin repel each other.
This attraction and repulsion is an electrical phenomenon, and the bodies that exhibit them are said to be electrified, or electrically charged. Bodies may be electrified in many other ways, as well as by friction. The electrical properties of the two pieces of glass are similar to each other but opposite to those of the two pieces of resin: The glass attracts what the resin repels and repels what the resin attracts.
If a body electrified in any manner whatsoever behaves as the glass does, that is, if it repels the glass and attracts the resin, the body is said to be vitreously electrified, and if it attracts the glass and repels the resin it is said to be resinously electrified. All electrified bodies are either vitreously or resinously electrified.
An established convention in the scientific community defines vitreous electrification as positive, and resinous electrification as negative. The exactly opposite properties of the two kinds of electrification justify our indicating them by opposite signs, but the application of the positive sign to one rather than to the other kind must be considered as a matter of arbitrary convention—just as it is a matter of convention in mathematical diagram to reckon positive distances towards the right hand.
No force, either of attraction or of repulsion, can be observed between an electrified body and a body not electrified.[22]
The role of charge in electric current
Electric current is the flow of electric charge through an object, which produces no net loss or gain of electric charge. The most common charge carriers are the positively charged proton and the negatively charged electron. The movement of any of these charged particles constitutes an electric current. In many situations, it suffices to speak of the conventional current without regard to whether it is carried by positive charges moving in the direction of the conventional current or by negative charges moving in the opposite direction. This macroscopic viewpoint is an approximation that simplifies electromagnetic concepts and calculations.
At the opposite extreme, if one looks at the microscopic situation, one sees there are many ways of carrying an electric current, including: a flow of electrons; a flow of electron holes that act like positive particles; and both negative and positive particles (ions or other charged particles) flowing in opposite directions in an electrolytic solution or a plasma.
Beware that, in the common and important case of metallic wires, the direction of the conventional current is opposite to the drift velocity of the actual charge carriers; i.e., the electrons. This is a source of confusion for beginners.
Flavour in particle physics |
|---|
Flavour quantum numbers |
|
Related quantum numbers |
|
Combinations |
|
Flavour mixing |
|
Conservation of electric charge
The total electric charge of an isolated system remains constant regardless of changes within the system itself. This law is inherent to all processes known to physics and can be derived in a local form from gauge invariance of the wave function. The conservation of charge results in the charge-current continuity equation. More generally, the rate of change in charge density ρ within a volume of integration V is equal to the area integral over the current density J through the closed surface S = ∂V, which is in turn equal to the net current I:
- −ddt∫VρdV=displaystyle -frac ddtint _Vrho ,mathrm d V=
 ∂Vdisplaystyle scriptstyle partial V
∂Vdisplaystyle scriptstyle partial VJ⋅dS=∫JdScosθ=I.displaystyle mathbf J cdot mathrm d mathbf S =int Jmathrm d Scos theta =I.
Thus, the conservation of electric charge, as expressed by the continuity equation, gives the result:
- I=−dQdt.displaystyle I=-frac mathrm d Qmathrm d t.
The charge transferred between times tidisplaystyle t_mathrm i 

- Q=∫titfIdtdisplaystyle Q=int _t_mathrm i ^t_mathrm f I,mathrm d t
where I is the net outward current through a closed surface and Q is the electric charge contained within the volume defined by the surface.
Relativistic invariance
Aside from the properties described in articles about electromagnetism, charge is a relativistic invariant. This means that any particle that has charge Q, no matter how fast it goes, always has charge Q. This property has been experimentally verified by showing that the charge of one helium nucleus (two protons and two neutrons bound together in a nucleus and moving around at high speeds) is the same as two deuterium nuclei (one proton and one neutron bound together, but moving much more slowly than they would if they were in a helium nucleus).[23][24][25]
See also
- SI electromagnetism units
- Color charge
- Partial charge
References
^ "CODATA Value: elementary charge". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2015. Retrieved 2015-09-22.2014 CODATA recommended values
.mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ ab "CIPM, 1946: Resolution 2". BIPM.
^ International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), ISBN 92-822-2213-6, archived (PDF) from the original on 2017-08-14, p. 150
^ Gambhir, RS; Banerjee, D; Durgapal, MC (1993). Foundations of Physics, Vol. 2. New Dehli: Wiley Eastern Limited. p. 51. Retrieved 10 October 2018.
^ Carron, Neal J. (21 May 2015). "Babel of units: The evolution of units systems in classical electromagnetism" (PDF). p. 5. Retrieved 31 March 2018.
^
Purcell, Edward M.; David J. Morin (2013). Electricity and Magnetism (3rd ed.). Cambridge University Press. p. 766. ISBN 9781107014022.
^ Roller, Duane; Roller, D.H.D. (1954). The development of the concept of electric charge: Electricity from the Greeks to Coulomb. Cambridge, MA: Harvard University Press. p. 1.
^ O'Grady, Patricia F. (2002). Thales of Miletus: The Beginnings of Western Science and Philosophy. Ashgate. p. 8. ISBN 1351895370.
^ ab Lives of the Eminent Philosophers by Diogenes Laërtius, Book 1, §24
^ Roller, Duane; Roller, D.H.D. (1953). "The Prenatal History of Electrical Science". American Journal of Physics. 21 (5): 348. Bibcode:1953AmJPh..21..343R. doi:10.1119/1.1933449.
^ Roller, Duane; Roller, D.H.D. (1953). "The Prenatal History of Electrical Science". American Journal of Physics. 21 (5): 351. Bibcode:1953AmJPh..21..343R. doi:10.1119/1.1933449.
^ Roller, Duane; Roller, D.H.D. (1953). "The Prenatal History of Electrical Science". American Journal of Physics. 21 (5): 353. Bibcode:1953AmJPh..21..343R. doi:10.1119/1.1933449.
^ ab Roller, Duane; Roller, D.H.D. (1953). "The Prenatal History of Electrical Science". American Journal of Physics. 21 (5): 356. Bibcode:1953AmJPh..21..343R. doi:10.1119/1.1933449.
^ Roche, J.J. (1998). The mathematics of measurement. London: The Athlone Press. p. 62. ISBN 0387915818.
^ Roller, Duane; Roller, D.H.D. (1954). The development of the concept of electric charge: Electricity from the Greeks to Coulomb. Cambridge, MA: Harvard University Press. p. 6–7.
Heilbron, J.L. (1979). Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics. University of California Press. p. 169. ISBN 978-0-520-03478-5.
^ Brother Potamian; Walsh, J.J. (1909). Makers of electricity. New York: Fordham University Press. p. 70.
^ Roller, Duane; Roller, D.H.D. (1954). The development of the concept of electric charge: Electricity from the Greeks to Coulomb. Cambridge, MA: Harvard University Press. p. 40.
^ Two Kinds of Electrical Fluid: Vitreous and Resinous – 1733. Charles François de Cisternay DuFay (1698–1739) Archived 2009-05-26 at the Wayback Machine.. sparkmuseum.com
^ Heilbron, J.L. (1979). Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics. University of California Press. pp. 280–289. ISBN 978-0-520-03478-5.
^ Roald K. Wangsness (1986) Electromagnetic Fields (2nd Ed.). Wiley.
ISBN 0-471-81186-6.
^ Denker, John (2007). "One Kind of Charge". www.av8n.com/physics. Archived from the original on 2016-02-05.
^ James Clerk Maxwell (1891) A Treatise on Electricity and Magnetism, pp. 32–33, Dover Publications
^ Jefimenko, O.D. (1999). "Relativistic invariance of electric charge" (PDF). Zeitschrift für Naturforschung A. 54 (10–11): 637–644. Bibcode:1999ZNatA..54..637J. doi:10.1515/zna-1999-10-1113. Retrieved 11 April 2018.
^ "How can we prove charge invariance under Lorentz Transformation?". physics.stackexchange.com. Retrieved 2018-03-27.
^ Singal, A.K. (1992). "On the charge invariance and relativistic electric fields from a steady conduction current". Physics Letters A. 162 (2): 91–95. Bibcode:1992PhLA..162...91S. doi:10.1016/0375-9601(92)90982-R. ISSN 0375-9601.
External links
- How fast does a charge decay?
- History of the electrical units.



