Nitrate
 | |
| Names | |
|---|---|
Systematic IUPAC name Nitrate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | NO− 3 |
Molar mass | 62.00 g·mol−1 |
Conjugate acid | Nitric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Nitrate is a polyatomic ion with the molecular formula NO−
3 and a molecular mass of 62.0049 u. Organic compounds that contain the nitrate ester as a functional group (RONO2) are also called nitrates.
Contents
1 Structure
2 Properties and diet
3 Occurrence
4 Uses
5 Detection
6 Toxicity
6.1 Poisoning
6.2 Human health effects
6.3 Aquatic toxicity
7 Domestic animal feed
8 Nitrate overview
9 See also
10 References
11 External links
Structure
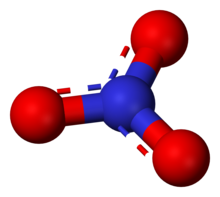
The anion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This results from a combination formal charge in which each of the three oxygens carries a −2⁄3 charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures:

Properties and diet

The nitrate ion with the partial charges shown
Almost all inorganic nitrate salts are soluble in water at standard temperature and pressure. A common example of an inorganic nitrate salt is potassium nitrate (saltpeter). A rich source of inorganic nitrate in the human body comes from diets rich in leafy green foods, such as spinach and arugula. NO−
3 (inorganic nitrate) is the viable active component within beetroot juice and other vegetables.
Dietary nitrate may be found in cured meats, various leafy vegetables, and drinking water; nitrite consumption is primarily determined by the amount of processed meats eaten, and the concentration of nitrates in these meats. Nitrate and water are converted in the body to nitric oxide, which could reduce hypertension. Anti-hypertensive diets, such as the DASH diet, typically contain high levels of nitrates, which are first reduced to nitrite in the saliva, as detected in saliva testing, prior to forming nitric oxide.[1]
Occurrence
Nitrate salts are found naturally on earth as large deposits, particularly of nitratine, a major source of sodium nitrate.
Nitrates are produced by a number of species of nitrifying bacteria, and the nitrate compounds for gunpowder (see this topic for more) were historically produced, in the absence of mineral nitrate sources, by means of various fermentation processes using urine and dung.
Nitrates are found in fertilizers.
As a byproduct of lightning strikes in earth's nitrogen-oxygen rich atmosphere, nitric acid is produced when nitrogen dioxide reacts with water vapor.
Uses

Table tennis balls are made from celluloid, an organic nitrate.
Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility and biodegradability. The main nitrate fertilizers are ammonium, sodium, potassium, and calcium salts. Several million kilograms are produced annually for this purpose.[2]
The second major application of nitrates is as oxidizing agents, most notably in explosives where the rapid oxidation of carbon compounds liberates large volumes of gases (see gunpowder for an example). Sodium nitrate is used to remove air bubbles from molten glass and some ceramics. Mixtures of the molten salt are used to harden some metals.
Explosives and table tennis balls are made from celluloid. In the early 20th century, most motion picture film was made of nitrocellulose, but the intense flammability of the film led to it being replaced with "safety film" by the mid-20th-century.
Although nitrites are the nitrogen compound chiefly used in meat curing, nitrates are used in certain specialty curing processes where a long release of nitrite from parent nitrate stores is needed. The use of nitrates in food preservation is controversial. This is due to the potential for the formation of nitrosamines when nitrates are present in high concentrations and the product is cooked at high temperatures.[3] The effect is seen for red or processed meat, but not for white meat or fish.[4][5] The production of carcinogenic nitrosamines may be inhibited by the use of the antioxidants vitamin C and the alpha-tocopherol form of vitamin E during curing.[6]
Under simulated gastric conditions, nitrosothiols rather than nitrosamines are the main nitroso species being formed.[4] The use of either compound is therefore regulated; for example, in the United States, the concentration of nitrates and nitrites is generally limited to 200 ppm or lower.[3] They are considered irreplaceable in the prevention of botulinum poisoning from consumption of cured dry sausages by preventing spore germination.[7]
Research has shown that dietary nitrate supplementation delivers positive results when testing endurance exercise performance.[8]
Detection
The historical standard method of testing for nitrate is the Cadmium Reduction Method, which is reliable and accurate although it is dependent on a toxic metal cadmium and thus not suitable for all applications. An alternative method for nitrate and nitrite analysis is enzymatic reduction using nitrate reductase,[9][10][11] which has recently been proposed by the US Environmental Protection Agency as an alternate test procedure for determining nitrate.[12] An open source photometer has been developed for this method to accurately detect nitrate in water, soils, forage, etc.[13]
Free nitrate ions in solution can be detected by a nitrate ion selective electrode. Such electrodes function analogously to the pH selective electrode. This response is partially described by the Nernst equation.
Toxicity
Poisoning
Nitrate poisoning can occur through enterohepatic metabolism of nitrate due to nitrite being an intermediate.[14] Nitrites oxidize the iron atoms in hemoglobin from ferrous iron(II) to ferric iron(III), rendering it unable to carry oxygen.[15] This process can lead to generalized lack of oxygen in organ tissue and a dangerous condition called methemoglobinemia. Although nitrite converts to ammonia, if there is more nitrite than can be converted, the animal slowly suffers from a lack of oxygen.[16]
Human health effects
Humans are subject to nitrate toxicity, with infants being especially vulnerable to methemoglobinemia. [17] Methemoglobinemia in infants is known as blue baby syndrome. Methemoglobin occurs in normal people in concentrations of 0.5-3.0%. [18]It is when concentrations of methemoglobin exceed 10% do clinical symptoms of methemoglobinemia occur. Any concentration above 50% can result in death. Through the Safe Drinking Water Act, the United States Environmental Protection Agency has set a maximum contaminant level of 10 mg/L or 10 ppm of nitrates in drinking water.[19] This particular standard was set to prevent methemoglobinemia in infants.[20] Infants exposed to water containing nitrates are at highest risk of developing blue baby syndrome during the first 6 months of life. [21] In the United States, it is estimated that 40,000 infants younger than 6 months live in homes with water contaminated with nitrates. [22] This is due to low concentrations of nitrate metabolizing triglycerides during this developmental period. Private water systems, such as well water in agricultural areas, are more likely to have nitrate levels above the maximum contaminant level (MCL). Unlike municipal water, well water is not treated and tested as often. Rural well-water near agricultural fields can become contaminated with nitrates due to manure, fertilizers or septic tanks. [22] Exposure commonly occurs when formula is mixed with well-water containing nitrates or infants under 6 months are fed vegetables washed with the contaminated drinking water. It is important to note that infants who are breastfed by mothers who ingest water with concentrations of nitrates (100 ppm) are not at risk of methemoglobinemia. [22] It is recommended that foods like green beans, carrots, spinach, squash and beets, are not fed to infants under 3 months.[22] These foods have naturally occurring nitrates which can be harmful to the infant. High levels of nitrate in fertilizer may also contribute to elevated levels of nitrate in the harvested plant. [23]
Not only are infants under 6 months a concern, but pregnant women with altered physiological states and compromised immune system can be at risk.[24] Pregnant women show a decrease in methemoglobin levels with increasing gestation.[25] Exposure to nitrate in groundwater during pregnancy at concentrations above the MCL was associated with increased risk for anencephaly.[26] A study done in Texas and Iowa found that mothers of babies with spina bifida were twice as likely to ingest 5 mg or more of nitrate daily from drinking water as mothers of babies without major birth defects. Mothers of babies with limb deficiencies, cleft palate, and cleft lip were, respectively, 1.8, 1.9 and 1.8 times more likely to ingest 5.42 mg or more of nitrate daily than mothers of babies without major birth defects. [27]
While there is evidence that shows nitrates can affect infants and pregnant women, recent evidence shows there are significant scientific doubts as to whether there is a causal link. [28][29] Blue baby syndrome now is thought to be the product of a number of other factors such as gastric upset, such as diarrheal infection, protein intolerance, heavy metal toxicity etc., with nitrates playing a minor role.
Some adults may be more susceptible to the effects of nitrates than others. In these adults, the methemoglobin reductase enzyme may be under-produced or absent in certain people who have an inherited mutation in the enzyme.[30] Such individuals cannot break down methemoglobin as rapidly as those who do have the enzyme, leading to increased circulating levels of methemoglobin (the implication being that their blood is not so oxygen-rich as that of the others). Diets rich in green, leafy vegetables typically accompany an increased nitrate intake. [31] A wide variety of medical conditions, such as food allergies, asthma, hepatitis, and gallstones, may be linked with low stomach acid; these individuals also may be highly sensitive to the effects of nitrate.[32]
Methemoglobinemia may be treated with methylene blue, which reduces ferric iron(III) back to ferrous iron(II) in affected blood cells.[33]
Another human health effects from the ingestion of nitrate is in the form of processed meat.[34] This form of ingestion may increase the risk pancreatic cancer. Processed meat can be cured with nitrate-based salt to decrease bacterial growth and improve flavor. When ingested, nitrate can become N-nitroso compounds (NOC), a probable human carcinogen. In a study performed by the US Government, there was a positive correlation between nitrate intake of more than 3g/day and pancreatic cancer in men. However, there are few data points which results in only a borderline significance.
Aquatic toxicity

Sea surface nitrate from the World Ocean Atlas
In freshwater or estuarine systems close to land, nitrate can reach concentrations that can potentially cause the death of fish. While nitrate is much less toxic than ammonia,[35] levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species.[36] However, in light of inherent problems with past protocols on acute nitrate toxicity experiments, the extent of nitrate toxicity has been the subject of recent debate.[37]
In most cases of excess nitrate concentrations in aquatic systems, the primary source is surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. This will contribute to eutrophication and can lead to algae blooms which may resilt in anoxia and the formation ofdead zones, these blooms may cause other changes to ecosystem function, favouring some groups of organisms over others. As a consequence, as nitrate forms a component of total dissolved solids, they are widely used as an indicator of water quality.
Domestic animal feed
Symptoms of nitrate poisoning in domestic animals include increased heart rate and respiration; in advanced cases blood and tissue may turn a blue or brown color. Feed can be tested for nitrate; treatment consists of supplementing or substituting existing supplies with lower nitrate material. Safe levels of nitrate for various types of livestock are as follows:[38]
| Category | %NO3 | %NO3–N | %KNO3 | Effects |
|---|---|---|---|---|
| 1 | <0.5 | <0.12 | <0.81 | Generally safe for beef cattle and sheep |
| 2 | 0.5–1.0 | 0.12–0.23 | 0.81–1.63 | Caution: some subclinical symptoms may appear in pregnant horses, sheep and beef cattle |
| 3 | 1.0 | 0.23 | 1.63 | High nitrate problems: death losses and abortions can occur in beef cattle and sheep |
| 4 | <1.23 | <0.28 | <2.00 | Maximum safe level for horses. Do not feed high nitrate forages to pregnant mares |
The values above are on a dry (moisture-free) basis.
Nitrate overview
Nitrate formation with elements of the periodic table.
Salts and covalent derivatives of the nitrate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
HNO3 | He | ||||||||||||||||||
LiNO3 | Be(NO3)2 | B(NO 3)− 4 | C | NO− 3, NH4NO3 | O | FNO3 | Ne | ||||||||||||
NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||||
KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 | Co(NO3)2, Co(NO3)3 | Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr | ||
RbNO3 | Sr(NO3)2 | Y | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 | ||
CsNO3 | Ba(NO3)2 | | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 | Tl(NO3)3, TlNO3 | Pb(NO3)2 | Bi(NO3)3 BiO(NO3) | Po | At | Rn | ||
FrNO3 | Ra(NO3)2 | | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||
| ↓ | |||||||||||||||||||
La(NO3)3 | Ce(NO3)3, Ce(NO3)4 | Pr | Nd | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
See also
- Ammonium
- f-ratio
- Nitrification
- Nitratine
- Peroxynitrate
- Sodium nitrate
References
^ Hord NG, Tang Y, Bryan NS (July 2009). "Food sources of nitrates and nitrites: the physiologic context for potential health benefits". The American Journal of Clinical Nutrition. 90 (1): 1–10. doi:10.3945/ajcn.2008.27131. PMID 19439460..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Laue W, Thiemann M, Scheibler E, Wiegand KW (2006). "Nitrates and Nitrites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_265.
^ ab "Curing Food". Edinformatics. Retrieved 21 February 2010.
^ ab Kuhnle GG, Bingham SA (November 2007). "Dietary meat, endogenous nitrosation and colorectal cancer". Biochemical Society Transactions. 35 (Pt 5): 1355–7. doi:10.1042/BST0351355. PMID 17956350.
^ Bingham SA, Hughes R, Cross AJ (November 2002). "Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response". The Journal of Nutrition. 132 (11 Suppl): 3522S–3525S. doi:10.1093/jn/132.11.3522S. PMID 12421881.
^ Parthasarathy DK, Bryan NS (November 2012). "Sodium nitrite: the "cure" for nitric oxide insufficiency". Meat Science. 92 (3): 274–9. doi:10.1016/j.meatsci.2012.03.001. PMID 22464105.
^ De Vries J (1997). Food Safety and Toxicity. CRC Press. p. 70. ISBN 978-0-8493-9488-1.
^ McMahon NF, Leveritt MD, Pavey TG (April 2017). "The Effect of Dietary Nitrate Supplementation on Endurance Exercise Performance in Healthy Adults: A Systematic Review and Meta-Analysis". Sports Medicine (Auckland, N.Z.). 47 (4): 735–756. doi:10.1007/s40279-016-0617-7. PMID 27600147.
^ Campbell WH, Song P, Barbier GG (28 March 2006). "Nitrate reductase for nitrate analysis in water". Environmental Chemistry Letters. 4 (2): 69–73. doi:10.1007/s10311-006-0035-4.
^ Patton CJ, Kryskalla JR (2016). "Analytical properties of some commercially available nitrate reductase enzymes evaluated as replacements for cadmium in automated, semiautomated, and manual colorimetric methods for determination of nitrate plus nitrite in water". Report No.: 2013–5033: U.S. Geological Survey Scientific Investigations Report. p. 366.
^ Patton CJ, Kryskalla JR (2011). "Colorimetric determination of nitrate plus nitrite in water by enzymatic reduction, automated discrete analyzer methods". U.S. Geological Survey Techniques and Methods: 34.
^ Federal Register. 2015; 80: 8962.
^ Wittbrodt BT, Squires DA, Walbeck J, Campbell E, Campbell WH, Pearce JM (2015). "Open-Source Photometric System for Enzymatic Nitrate Quantification". Plos One. 10 (8): e0134989. doi:10.1371/journal.pone.0134989. PMC 4526554. PMID 26244342.
^ "Nitrate and Nitrite Poisoning: Introduction". The Merck Veterinary Manual. Retrieved 2008-12-27.
^ Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N (January 2005). "The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation". Journal of Inorganic Biochemistry. 99 (1): 237–46. doi:10.1016/j.jinorgbio.2004.10.034. PMID 15598504.
^ Stoltenow C, Lardy G (May 2008). "Nitrate Poisoning of Livestock" (PDF). North Dakota State University. pp. 1–4. Retrieved October 30, 2013.
^ Greer FR, Shannon M (September 2005). "Infant methemoglobinemia: the role of dietary nitrate in food and water". Pediatrics. 116 (3): 784–6. doi:10.1542/peds.2005-1497. PMID 16140723.
^ Read "Nitrate and Nitrite in Drinking Water" at NAP.edu.
^ "4. What are EPA's drinking water regulations for nitrate?". Ground Water & Drinking Water. Retrieved 2018-11-13.
^ ATSDR. "Nitrate/Nitrite Toxicity 2013: U.S. Standards and Regulations for Nitrates and Nitrites Exposure | ATSDR - Environmental Medicine & Environmental Health Education - CSEM". www.atsdr.cdc.gov. Retrieved 2018-11-15.
^ Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (July 2000). "Blue babies and nitrate-contaminated well water". Environmental Health Perspectives. 108 (7): 675–8. doi:10.1289/ehp.00108675. PMC 1638204. PMID 10903623.
^ abcd Preboth, Monica (2005-12-15). "AAP Clinical Report on Infant Methemoglobinemia". American Family Physician. 72 (12): 2558. ISSN 1532-0650.
^ Manassaram DM, Backer LC, Messing R, Fleming LE, Luke B, Monteilh CP (October 2010). "Nitrates in drinking water and methemoglobin levels in pregnancy: a longitudinal study". Environmental Health. 9 (1): 60. doi:10.1186/1476-069x-9-60. PMC 2967503. PMID 20946657.
^ Tabacova S, Balabaeva L, Little RE (1997). "Maternal exposure to exogenous nitrogen compounds and complications of pregnancy". Archives of Environmental Health. 52 (5): 341–7. doi:10.1080/00039899709602209. PMID 9546756.
^ Manassaram DM, Backer LC, Messing R, Fleming LE, Luke B, Monteilh CP (October 2010). "Nitrates in drinking water and methemoglobin levels in pregnancy: a longitudinal study". Environmental Health. 9: 60. doi:10.1186/1476-069X-9-60. PMC 2967503. PMID 20946657.
^ Croen LA, Todoroff K, Shaw GM (February 2001). "Maternal exposure to nitrate from drinking water and diet and risk for neural tube defects". American Journal of Epidemiology. 153 (4): 325–31. doi:10.1093/aje/153.4.325. PMID 11207149.
^ "Higher daily nitrate intake from drinking water during pregnancy associated with birth defects". Vital Record. 2014-06-02. Retrieved 2018-12-06.
^ T. M. Addiscott & N. Benjamin: Nitrate and human health, Soil Use and Management, Volume 20, Issue 2, pages 98–104, June 2004
^ A. A. Avery: Infant Methemoglobinemia - Reexamining the Role of Drinking Water Nitrates, Environmental Health Perspectives, Volume 107, Number 7, July 1999
^ "Q&A: Nitrate in Drinking Water" (in English and Spanish). Washington State Department of Health. DOH-331-214. Retrieved 9 June 2013.
^ "Nitrate in drinking water :: Washington State Department of Health". www.doh.wa.gov. Retrieved 2018-12-06.
^ "Heartburn and Asthma: What's the Link?". WebMD. Retrieved 2018-12-06.
^ "Methemoglobinemia". The New York Times. Retrieved 25 October 2016.
^ Quist AJ, Inoue-Choi M, Weyer PJ, Anderson KE, Cantor KP, Krasner S, Freeman LE, Ward MH, Jones RR (January 2018). "Ingested nitrate and nitrite, disinfection by-products, and pancreatic cancer risk in postmenopausal women". International Journal of Cancer. 142 (2): 251–261. doi:10.1002/ijc.31055. PMC 5788281. PMID 28921575.
^ Romano N, Zeng C (September 2007). "Acute toxicity of sodium nitrate, potassium nitrate, and potassium chloride and their effects on the hemolymph composition and gill structure of early juvenile blue swimmer crabs(Portunus pelagicus Linnaeus, 1758) (Decapoda, Brachyura, Portunidae)". Environmental Toxicology and Chemistry. 26 (9): 1955–62. doi:10.1897/07-144r.1. PMID 17705664.
^ Sharpe, Shirlie. "Nitrates in the Aquarium". About.com. Retrieved October 30, 2013.
^ Romano N, Zeng C (December 2007). "Effects of potassium on nitrate mediated alterations of osmoregulation in marine crabs". Aquatic Toxicology. 85 (3): 202–8. doi:10.1016/j.aquatox.2007.09.004. PMID 17942166.
^ "Nitrate Risk in Forage Crops - Frequently Asked Questions". Agriculture and Rural Development. Government of Alberta. Retrieved October 30, 2013.
External links
| Wikimedia Commons has media related to nitrates. |
- ATSDR - Case Studies in Environmental Medicine - Nitrate/Nitrite Toxicity