Sulfate
 | |||
| |||
| Names | |||
|---|---|---|---|
IUPAC name Sulfate | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
ChEBI |
| ||
ChemSpider |
| ||
ECHA InfoCard | 100.108.048 | ||
EC Number | 233-334-2 | ||
PubChem CID |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | SO2− 4 | ||
Molar mass | 96.06 g·mol−1 | ||
Conjugate acid | Hydrogen sulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
The sulfate or sulphate (see spelling differences) ion is a polyatomic anion with the empirical formula SO2−
4. Sulfate is the spelling recommended by IUPAC, but sulphate is used in British English. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
Contents
1 Structure
2 Bonding
3 Preparation
4 Properties
5 Uses and occurrence
5.1 Commercial applications
5.2 Occurrence in nature
6 History
7 Environmental effects
7.1 Main effects on climate
8 Hydrogen sulfate (bisulfate)
9 Other sulfur oxyanions
10 Notes
11 See also
12 References
Structure
The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogen sulfate) ion, HSO−
4, which is in turn the conjugate base of H
2SO
4, sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of the sulfate ion is as predicted by VSEPR theory.
Bonding

Two models of the sulfate ion.
1 with polar covalent bonds only; 2 with an ionic bond

Six resonances
The first description of the bonding in modern terms was by Gilbert Lewis in his groundbreaking paper of 1916 where he described the bonding in terms of electron octets around each atom, that is no double bonds and a formal charge of +2 on the sulfur atom.[1][a]
Later, Linus Pauling used valence bond theory to propose that the most significant resonance canonicals had two pi bonds involving d orbitals. His reasoning was that the charge on sulfur was thus reduced, in accordance with his principle of electroneutrality.[2] The S−O bond length of 149 pm is shorter than the bond lengths in sulfuric acid of 157 pm for S−OH. The double bonding was taken by Pauling to account for the shortness of the S−O bond. Pauling's use of d orbitals provoked a debate on the relative importance of π bonding and bond polarity (electrostatic attraction) in causing the shortening of the S−O bond. The outcome was a broad consensus that d orbitals play a role, but are not as significant as Pauling had believed.[3][4]
A widely accepted description involving pπ – dπ bonding was initially proposed by D. W. J. Cruickshank. In this model, fully occupied p orbitals on oxygen overlap with empty sulfur d orbitals (principally the dz2 and dx2–y2).[5] However, in this description, despite there being some π character to the S−O bonds, the bond has significant ionic character. For sulfuric acid, computational analysis (with natural bond orbitals) confirms a clear positive charge on sulfur (theoretically +2.45) and a low 3d occupancy. Therefore, the representation with four single bonds is the optimal Lewis structure rather than the one with two double bonds (thus the Lewis model, not the Pauling model).[6] In this model, the structure obeys the octet rule and the charge distribution is in agreement with the electronegativity of the atoms. The discrepancy between the S−O bond length in the sulfate ion and the S−OH bond length in sulfuric acid is explained by donation of p-orbital electrons from the terminal S=O bonds in sulfuric acid into the antibonding S−OH orbitals, weakening them resulting in the longer bond length of the latter.
However, the bonding representation of Pauling for sulfate and other main group compounds with oxygen is still a common way of representing the bonding in many textbooks.[5][7] The apparent contradiction can be cleared if one realizes that the covalent double bonds in the Lewis structure in reality represent bonds that are strongly polarized by more than 90% towards the oxygen atom. On the other hand, in the structure with an dipolar bond, the charge is localized as a lone pair on the oxygen.[6]
Preparation
Methods of preparing metal sulfates include:[7]
- treating metal, metal hydroxide or metal oxide with sulfuric acid
- Zn + H2SO4 → ZnSO4 + H2
- Cu(OH)2 + H2SO4 → CuSO4 + 2 H2O
- CdCO3 + H2SO4 → CdSO4 + H2O + CO2
- oxidation of metal sulfides or sulfites
Properties
Many examples of ionic sulfates are known, and many of these are highly soluble in water. Exceptions include calcium sulfate, strontium sulfate, lead(II) sulfate, and barium sulfate, which are poorly soluble. Radium sulfate is the most insoluble sulfate known. The barium derivative is useful in the gravimetric analysis of sulfate: if one adds a solution of, perhaps, barium chloride to a solution containing sulfate ions, the appearance of a white precipitate, which is barium sulfate, indicates that sulfate anions are present.
The sulfate ion can act as a ligand attaching either by one oxygen (monodentate) or by two oxygens as either a chelate or a bridge.[7] An example is the complex [Co(en)2(SO4)]+Br−[7] or the neutral metal complex PtSO4(P(C6H5)3)2 where the sulfate ion is acting as a bidentate ligand. The metal–oxygen bonds in sulfate complexes can have significant covalent character.
Uses and occurrence
Commercial applications

Knapsack sprayer used to apply sulfate to vegetables. Valencian Museum of Ethnology.
Sulfates are widely used industrially. Major compounds include:
Gypsum, the natural mineral form of hydrated calcium sulfate, is used to produce plaster. About 100 million tonnes per year are used by the construction industry.
Copper sulfate, a common algaecide, the more stable form (CuSO4) is used for galvanic cells as electrolyte
Iron(II) sulfate, a common form of iron in mineral supplements for humans, animals, and soil for plants
Magnesium sulfate (commonly known as Epsom salts), used in therapeutic baths
Lead(II) sulfate, produced on both plates during the discharge of a lead–acid battery
Sodium Laureth Sulfate, or SLES, a common detergent in shampoo formulations
Occurrence in nature
Sulfate-reducing bacteria, some anaerobic microorganisms, such as those living in sediment or near deep sea thermal vents, use the reduction of sulfates coupled with the oxidation of organic compounds or hydrogen as an energy source for chemosynthesis.
History
Some sulfates were known to alchemists. The vitriol salts, from the Latin vitreolum, glassy, were so-called because they were some of the first transparent crystals known.[8]Green vitriol is iron(II) sulfate heptahydrate, FeSO4·7H2O; blue vitriol is copper(II) sulfate pentahydrate, CuSO4·5H2O and white vitriol is zinc sulfate heptahydrate, ZnSO4·7H2O. Alum, a double sulfate of potassium and aluminium with the formula K2Al2(SO4)4·24H2O, figured in the development of the chemical industry.
Environmental effects
Sulfates occur as microscopic particles (aerosols) resulting from fossil fuel and biomass combustion. They increase the acidity of the atmosphere and form acid rain. The anaerobic sulfate-reducing bacteria Desulfovibrio desulfuricans and D. vulgaris can remove the black sulfate crust that often tarnishes buildings.[9]
Main effects on climate
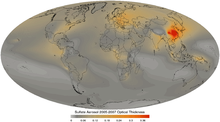
Sulfate aerosol optical thickness 2005 to 2007 average
The main direct effect of sulfates on the climate involves the scattering of light, effectively increasing the Earth's albedo. This effect is moderately well understood and leads to a cooling from the negative radiative forcing of about 0.4 W/m2 relative to pre-industrial values,[10] partially offsetting the larger (about 2.4 W/m2) warming effect of greenhouse gases. The effect is strongly spatially non-uniform, being largest downstream of large industrial areas.[11]
The first indirect effect is also known as the Twomey effect. Sulfate aerosols can act as cloud condensation nuclei and this leads to greater numbers of smaller droplets of water. Lots of smaller droplets can diffuse light more efficiently than just a few larger droplets.
The second indirect effect is the further knock-on effects of having more cloud condensation nuclei. It is proposed that these include the suppression of drizzle, increased cloud height,[12][full citation needed] to facilitate cloud formation at low humidities and longer cloud lifetime.[13][full citation needed] Sulfate may also result in changes in the particle size distribution, which can affect the clouds radiative properties in ways that are not fully understood. Chemical effects such as the dissolution of soluble gases and slightly soluble substances, surface tension depression by organic substances and accommodation coefficient changes are also included in the second indirect effect.[14]
The indirect effects probably have a cooling effect, perhaps up to 2 W/m2, although the uncertainty is very large.[15][full citation needed] Sulfates are therefore implicated in global dimming. Sulfate is also the major contributor to stratospheric aerosol formed by oxidation of sulfur dioxide injected into the stratosphere by impulsive volcanoes such as the 1991 eruption of Mount Pinatubo in the Philippines. This aerosol exerts a cooling effect on climate during its 1-2 year lifetime in the stratosphere.
Hydrogen sulfate (bisulfate)
 | |
| Names | |
|---|---|
IUPAC name Hydrogen sulfate | |
| Other names Bisulfate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
ECHA InfoCard | 100.108.048 |
SMILES
| |
| Properties | |
Chemical formula | HSO− 4 |
Molar mass | 97.071 g/mol |
Melting point | 270.47 °C (518.85 °F; 543.62 K) |
Boiling point | 623.89 °C (1,155.00 °F; 897.04 K) |
Vapor pressure | 0.00791 Pa (5.93E-005 mm Hg) |
Conjugate acid | Sulfuric acid |
Conjugate base | Sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
The conjugate base of sulfuric acid (H2SO4)—a dense, colourless, oily, corrosive liquid—is the hydrogen sulfate ion (HSO−
4), also called the bisulfate ion.[b] Sulfuric acid is classified as a strong acid; in aqueous solutions it ionizes completely to form hydronium ions (H3O+) and hydrogen sulfate (HSO−
4). In other words, the sulfuric acid behaves as a Brønsted–Lowry acid and is deprotonated. Bisulfate has a molar mass of 97.078 g/mol. It has a valency of 1. An example of a salt containing the HSO−
4 group is sodium bisulfate, NaHSO4. In dilute solutions the hydrogen sulfate ions also dissociate, forming more hydronium ions and sulfate ions (SO2−
4). The CAS registry number for hydrogen sulfate is 14996-02-2.
Other sulfur oxyanions
| Molecular formula | Name |
|---|---|
| SO2− 5 | Peroxomonosulfate |
| SO2− 4 | Sulfate |
| SO2− 3 | Sulfite |
| S 2O2− 8 | Peroxydisulfate |
| S 2O2− 7 | Pyrosulfate |
| S 2O2− 6 | Dithionate |
| S 2O2− 5 | Metabisulfite |
| S 2O2− 4 | Dithionite |
| S 2O2− 3 | Thiosulfate |
| S 3O2− 6 | Trithionate |
| S 4O2− 6 | Tetrathionate |
Notes
^ Lewis assigned to sulfur a negative charge of two, starting from six own valence electrons and ending up with eight electrons shared with the oxygen atoms. In fact, sulfur donates two electrons to the oxygen atoms.
^ The prefix "bi" in "bisulfate" comes from an outdated naming system and is based on the observation that there is twice as much sulfate (SO2−
4) in sodium bisulfate (NaHSO4) and other bisulfates as in sodium sulfate (Na2SO4) and other sulfates. See also bicarbonate.
See also
| Wikimedia Commons has media related to sulfates. |
- Sulfonate
- Sulfation and desulfation of lead–acid batteries
- Sulfate-reducing microorganisms
References
^ Lewis, Gilbert N. (1916). "The Atom and the Molecule". J. Am. Chem. Soc. 38: 762–785. doi:10.1021/ja02261a002..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em (See page 778.)
^ Pauling, Linus (1948). "The modern theory of valency". J. Chem. Soc.: 1461–1467. doi:10.1039/JR9480001461.
^ Coulson, C. A. (1969). "d Electrons and Molecular Bonding". Nature. 221: 1106. Bibcode:1969Natur.221.1106C. doi:10.1038/2211106a0.
^ Mitchell, K. A. R. (1969). "Use of outer d orbitals in bonding". Chem. Rev. 69: 157. doi:10.1021/cr60258a001.
^ ab Cotton, F. Albert; Wilkinson, Geoffrey (1966). Advanced Inorganic Chemistry (2nd ed.). New York, NY: Wiley.
^ ab Stefan, Thorsten; Janoschek, Rudolf (Feb 2000). "How relevant are S=O and P=O Double Bonds for the Description of the Acid Molecules H2SO3, H2SO4, and H3PO4, respectively?". J. Mol. Modeling. 6 (2): 282–288. doi:10.1007/PL00010730.
^ abcd Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
^ Taylor, F. Sherwood (1942). Inorganic and Theoretical Chemistry (6th ed.). William Heinemann.
^ Andrea Rinaldi (Nov 2006). "Saving a fragile legacy. Biotechnology and microbiology are increasingly used to preserve and restore the worlds cultural heritage". EMBO Reports. 7 (11): 1075–1079. doi:10.1038/sj.embor.7400844. PMC 1679785. PMID 17077862.
^ Intergovernmental Panel on Climate Change (2007). "Chapter 2: Changes in Atmospheric Constituents and Radiative Forcing". Working Group I: The Scientific Basis.
^ Sulfate distribution in the atmosphere (Map).
^ Pincus & Baker 1994
^ Albrecht 1989
^ Rissman, T. A.; Nenes, A.; Seinfeld, J. H. "Chemical Amplification (or dampening) of the Twomey Effect: Conditions derived from droplet activation theory" (PDF).
^ Archer, David. Understanding the Forecast. p. 77. Figure 10.2
